How To Find Efficiency Of An Engine
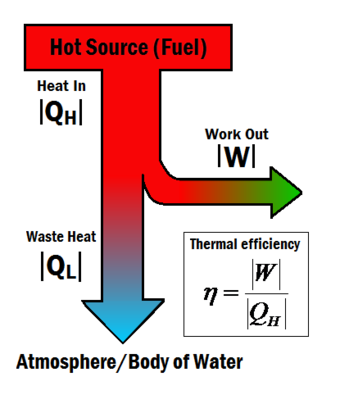
Figure ane: The corporeality of work output for a given amount of heat gives a system its thermal efficiency.[one]
Heat engines turn estrus into work. The thermal efficiency expresses the fraction of heat that becomes useful work. The thermal efficiency is represented by the symbol [math]\eta[/math], and tin be calculated using the equation:
Where:
[math]W[/math] is the useful work and
[math]Q_H[/math] is the total oestrus energy input from the hot source.[2]
Heat engines frequently operate at around 30% to fifty% efficiency, due to practical limitations. It is impossible for heat engines to accomplish 100% thermal efficiency ([math]\eta = 1[/math]) according to the Second law of thermodynamics. This is impossible because some waste heat is always produced produced in a heat engine, shown in Effigy 1 by the [math]Q_L[/math] term. Although consummate efficiency in a heat engine is impossible, at that place are many means to increase a system'southward overall efficiency.
An Example
If 200 joules of thermal energy as heat is input ([math]Q_H[/math]), and the engine does eighty J of piece of work ([math]W[/math]), so the efficiency is 80J/200J, which is 40% efficient.
This same outcome can exist gained by measuring the waste rut of the engine. For case, if 200 J is put into the engine, and discover 120 J of waste material heat, then 80 J of work must take been washed, giving 40% efficiency.
Carnot Efficiency
- main article
There is a maximum accessible efficiency of a estrus engine which was derived by physicist Sadi Carnot. Post-obit laws of thermodynamics the equation for this turns out to exist
Where
[math]T_L[/math] is the temperature of the cold 'sink' and
[math]T_H[/math] is the temperature of the heat reservoir.
This describes the efficiency of an idealized engine, which in reality is impossible to accomplish.[three] From this equation, the lower the sink temperature [math]T_L[/math] or the college the source temperature [math]T_H[/math], the more piece of work is available from the oestrus engine. The energy for piece of work comes from a subtract in the full free energy of the fluid used in the system. Therefore the greater the temperature modify, the greater this decrease in the fluid and thus the greater energy available to do work is.[iv]
For Further Reading
For further data please meet the related pages beneath:
- Oestrus engine
- Hydrocarbon combustion is frequently the source of oestrus for these engines.
- Solar thermal ability plant
- Nuclear power plant
- For thermal efficiency of car engines, click hither
- Or explore a random page!
References
- ↑ This flick was made by the Energy Education team.
- ↑ TPUB Engine Mechanics. (April 4, 2015). Thermal Efficiency [Online]. Bachelor: http://enginemechanics.tpub.com/14075/css/14075_141.htm
- ↑ Hyperphysics, Carnot Cycle [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/carnot.html
- ↑ R. A. Hinrichs and M. Kleinbach, "Estrus and Work," in Free energy: Its Use and the Environs, quaternary ed. Toronto, Ont. Canada: Thomson Brooks/Cole, 2006, ch.4, sec.E, pp.115
Source: https://energyeducation.ca/encyclopedia/Thermal_efficiency
Posted by: faydoely1954.blogspot.com


0 Response to "How To Find Efficiency Of An Engine"
Post a Comment